Many esters have "fruity" odors as you experienced in an earlier experiment with methyl salicylate, commonly referred to as oil of wintergreen.
They are commonly used as artificial
flavors and fragrances. Flavor is a perceived quality that results from a
combination of taste and odor transmitted by receptors on the tongue (taste
buds) and nose (olfactory bulb). Natural flavors and odors are complicated
mixtures of odorants and are not exactly matched by a single compound.
However, most people cannot taste a difference
between natural and artificial flavorings in food products.
Although the “fruity” tastes and odors of esters are pleasant,
they are not widely used in high-quality perfumes and colognes, but more
often in the inexpensive “toilet
waters.” One reason is the quality of the scent. Another is
the hydrolytic reactivity of the ester functional group, which was the reaction
that you employed in the synthesis of
salicylic acid
from methyl salicylate. Esters hydrolyze under basic or acidic conditions.
Sweat (perspiration) tends to be acidic, having a pH < 7, which catalyzes
ester hydrolysis to the corresponding carboxylic acid and
alcohol. Low molecular weight carboxylic acids
are typically not very pleasant to smell as you experienced in the first
Chem 226 experiment. One such acid butanoic acid,
has a strong odor like that of rancid butter and is one of the components
of sweat and body odor.
Have you ever noticed a person wearing "cheap" perfume or cologne, who
reeked of a smell that seemed to be both sweet and awful at the same time?
Attempts to cover body odor with an inexpensive scent will make the problem
worse....
unless
you never sweat.
A sweet, fruity odor can also serve a plant as an allomone (a chemical that
communicates with and alters the behavior of other species). The fruity
odor can attract
insects that assist in pollenation by transporting pollen to
other plants.
However, the allomone of one species may also serve as a pheromone in another.
Remember, pheromones are chemicals that serve to communicate
with and alter the behavior of the same species, as you saw in the earlier
on-line Chemical Communication structural
exercises. Isopentyl acetate, which smells like banana, is an allomone
and is also an alarm
pheromone of the honey bee. When a honey bee stings, it looses its stinger
and dies. It also releases alarm pheromones in the venom; this includes
isopentyl acetate. The ester provokes other bees to swarm and
attack the intruder. ........ Do bee keepers ever risk wearing perfume
or after shave?
In this experiment you will prepare an acetate ester from acetic
acid and an alcohol. The reaction is catalyzed
by sulfuric acid, but the catalyst affects only the rate of reaction, and
not the extent of reaction. The desired equilibrium product is present in
significant amounts only if the equilibrium constant is favorably large.
The equilibrium constant for this reaction is rather small
(<4). Therefore, simply mixing
equal molar amounts of the starting materials will convert only about 50-60%
of the starting material into product. But, recall Le Chatelier's principle,
there are two ways to adjust reagent concentrations to force the alcohol
to produce ester.
One way is to remove product as it forms. The other way is to use a large
excess
of acetic acid. This experiment is based on the
latter approach, but it raises three issues: cost, availability, and by-products/disposal.
Acetic acid is inexpensive and readily available. Consider the reaction's
procedure and by-product disposal, how "green" is this procedure?
Procedure: (Budget 2.0 lab periods)
Complete the following table and place in your lab notebook under the Physical Constants Data section for this experiment.
Structure |
Molecular Formula |
Molar mass(g/mol) |
b.p. oC |
d g/mL |
Safety |
|
| acetic acid | 60.1 |
118 |
1.049 |
x |
||
| alcohol | ||||||
| ester | ||||||
| sulfuric acid | 98.1 |
290 |
1.84 |
x |
|
Your synthetic target is to prepare 5.00g (theoretical) of pure ester, assuming 100% yield. Calculate the amount of starting alcohol and acetic acid in both grams and mL that you will need to start with as part of your prelab. Clearly show your calculation to include millimoles, grams, milliliters of alcohol and glacial acetic acid respectively that you plan to use and complete the prelab using the following experimental instructions; show your notebook to Dr. R. for initialing.
Reflux
Combine the correct amount of alcohol, acetic acid (3 molar
equivalents), and concentrated sulfuric acid (1 mL) in a clean,
dry,
round bottom flask. Put a boiling chip in your flask and attach a reflux
condenser. Reflux your mixture for 30-40 minutes while occasionally shaking
the apparatus carefully, then stop heating the mixture and allow it to
cool to room temperature.
Work up
Pour the mixture into a separatory funnel. Add 10 mL of water. Mix the layers
by carefully shaking. Separate the layers and wash the organic layer with
3 x 5 mL portions of 5% NaHCO3. [NOTE: Residual acetic acid and
sulfuric
acid will
react vigorously
with NaHCO3 and release lots of gas. Don't mix your layers until
the gas evolution appears to have stopped, then mix them cautiously (and
vent the
funnel frequently).] Wash the organic layer with 2 x 5 mL of saturated NaCl
solution. Dispose of the aqueous layers in the acid/neutralized-waste sink
witth some solid sodium bicarbonate added.
Dry the organic layer with 1-2 g of of anhydrous Na2SO4.
Gravity filter the solution to remove the Na2SO4 and
distill the product.
Simple
Distillation
Purification calls for a simple macroscale distillation at
atmospheric pressure of the acetate
ester.
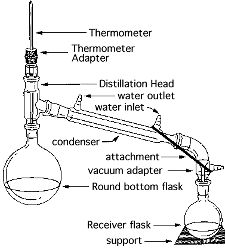
Collect a low boiling fraction as necessary. Prepare one clean, pre-weighed (tared) collection flask for the distillate corresponding to acetate.
NOTE: You and your partner have been assigned one low boiling ester and one high boiling ester. Distill only the lower boiling ester and then proceed to the next part IR-Characterization/ Index of Refraction. For the higher boiling ester continue directly on to the next part: IR-Characterization/ Index of Refraction.
IR-Characterization/ Index
of Refraction
Weigh your product. Calculate the % yield. Determine
its index of refraction.
1) Refer to IR-Tutor & tutorial: http://wwwchem.csustan.edu/Tutorials/INFRARED.HTM
2) Complete the worksheet, Infrared Analysis (IR) / Synthesis of Acetates .pdf : Unknown spectra (Identify the chemical function present in each Web unknown assigned to you. If your DVC ID ends in an odd number, do the odd Unknowns; if it ends in an even number or zero, do the even. Then, find a partner who has done the other set and explain your assignments of functions and peaks in the spectra that support your choices. Complete the entire form of 10 unknowns. The selection is limited to alcohols, carboxylic acids, esters, ethers, ketones and aldehydes. Be sure to provide the key peak(s) in the spectrum that support your assignment of the function. [NOTE: The molecules contain only C,H,O; there are no nitrogen atoms in the molecules.]
3) Run FT-IR spectra on 1) your individual unknown liquid (See: Class ASSIGNMENTS) and 2) the ester that you synthesized and distilled.
4) Analyze the spectra. a. What function is present in the liquid unknown? b. Consider the starting alcohol and glacial acetic acid: are either or both of them present as impurities in your acetate product? c. What peaks indicate the presence of product (ester)? Are the peaks in either of the reactants?
[Analyze the distilled product for impurities using gas chromatography if instructed to do so and as time allows.]
Answer the Post lab questions:
a) Major
League Baseball & Chewing Gum
b) Complete Odor,
Functions & Structures .pdf (Post
lab); refer to the review article on reserve in the DVC library.
"Structure-Odor Relationships", Karen J. Rossiter, Chem.
Rev., 96, 3201-3240, 1996 (Library Reserve under Dr. R's name.) Reading: pp.
3201-08, 3216-26. [For a better view of the rose oxide isomers see: rose
oxide #1 and rose
oxide #2. Rose oxide #1 is ~100x more powerful in its scent than #2.]